Accountability
Pfizer agrees to let other companies make it’s COVID-19 pill

Pfizer Inc. has signed a deal with a U.N.-backed group to allow other manufacturers to make its experimental COVID-19 pill, a move that could make the treatment available to more than half of the world’s population.
In a statement issued Tuesday, Pfizer said it would grant a license for the antiviral pill to the Geneva-based Medicines Patent Pool, which would let generic drug companies produce the pill for use in 95 countries, making up about 53% of the world’s population.
The deal excludes some large countries that have suffered devastating coronavirus outbreaks. For example, while a Brazilian company could get a license to make the pill for export to other countries, the medicine could not be made generically for the use in Brazil.
Still, health officials said the fact that the deal was struck even before Pfizer’s pill has been authorized anywhere, could help to end the pandemic quicker.
Esteban Burrone, head of policy at the Medicines Patent Pool, said, “It’s quite significant that we will be able to provide access to a drug that appears to be effective and has just been developed, to more than 4 billion people.” He estimated that other drugmakers would be able to start producing the pill within months, but acknowledged the agreement wouldn’t please everyone.
Burrone said, “We try to strike a very delicate balance between the interests of the (company), the sustainability required by generic producers and most importantly, the public health needs in lower and middle-income countries.”
Under the terms of the agreement, Pfizer will not receive royalties on sales in low-income countries and will waive royalties on sales in all countries covered by the agreement while COVID-19 remains a public health emergency.
Earlier this month, Pfizer said its pill cut the risk of hospitalization and death by nearly 90% in people with mild to moderate coronavirus infections. Independent experts recommended halting the company’s study based on its promising results.
Pfizer said it would ask the U.S. Food and Drug Administration and other regulators to authorize the pill as soon as possible. Since the pandemic erupted last year, researchers worldwide have raced to develop a pill to treat COVID-19 that can be taken at home easily to ease symptoms, speed recovery and keep people out of the hospital. At the moment, most COVID-19 treatments must be delivered intravenously or by injection.
The decisions by Pfizer and Merck to share their COVID-19 drug patents stands in stark contrast to reusal of Pfizer and other vaccine-makers to release their vaccine recipes for wider production. A hub set up by the World Health Organization in South Africa intended to share messenger RNA vaccine recipes and technologies has not enticed a single pharmaceutical to join.
Fewer than 1% of Pfizer’s COVID-19 shots have gone to poorer countries. Robbie Silverman of Oxfam America welcomed Pfizer’s agreement to let other makers produce its COVID antivirus,, but he noted that billions would still be left without access, including to the company’s vaccine.
Silverman said, “This move also begs the important question: If Pfizer can share data and intellectual property on a medicine, why have they so far categorically reused to do so for their COVID vaccine” (ABC News).
Terry A. Hurlbut has been a student of politics, philosophy, and science for more than 35 years. He is a graduate of Yale College and has served as a physician-level laboratory administrator in a 250-bed community hospital. He also is a serious student of the Bible, is conversant in its two primary original languages, and has followed the creation-science movement closely since 1993.
-

 Civilization4 days ago
Civilization4 days agoVance Defends Operation Epic Fury After Years of Opposing Interventions
-
 Civilization2 days ago
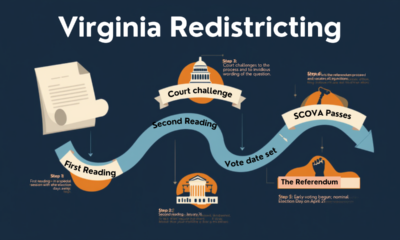
Civilization2 days agoVirginia redistricting – the forgotten theater
-

 Education4 days ago
Education4 days agoWaste of the Day: DEI Contractors Remain in Military’s K-12 Schools
-

 Executive5 days ago
Executive5 days agoWaste of the Day: Throwback Thursday – Fees Paid For Empty Bank Accounts
-

 Civilization3 days ago
Civilization3 days agoTrump’s SOTU Speech a Win, But It’s Not Enough
-
 Civilization4 days ago
Civilization4 days agoAt a Time of International Turmoil, ARC-ES Can Bring Energy Stability to the U.S.
-

 Civilization2 days ago
Civilization2 days agoWhat the Political Attacks on Fetterman Reveal
-

 Clergy2 days ago
Clergy2 days agoDecapitating Amalek: Iran, Purim, and the Obligation to Act in Time


