Accountability
FDA authorizes Pfizer booster shots for 12- to 15-year-olds
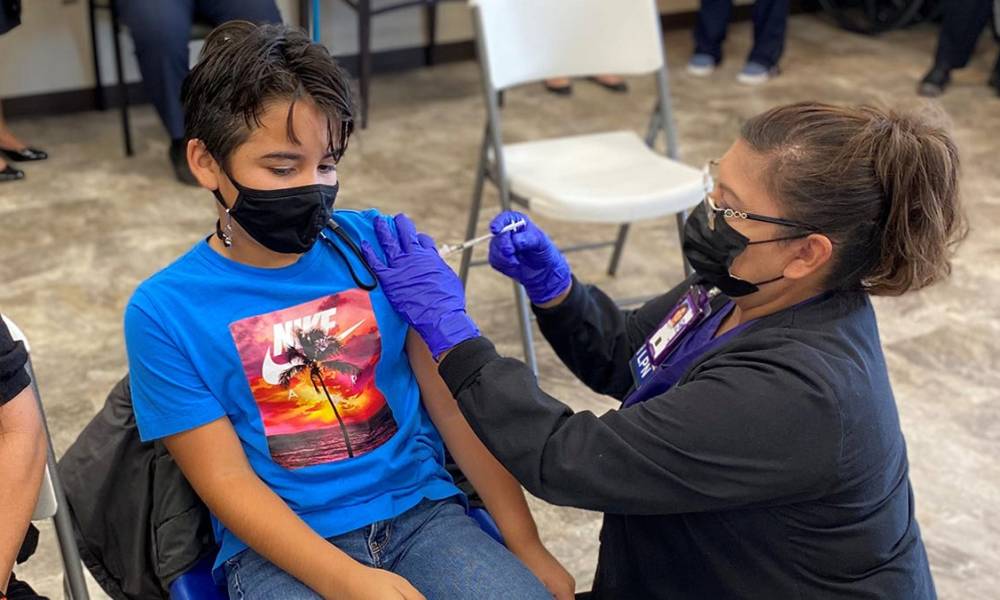
On Monday, the Food and Drug Administration expanded authorization for emergency use of a booster dose of the Pfizer-BioNTech coronavirus vaccine so that it now includes 12- to 15-year-olds.
“Hopefully this will be not just a call for people to get their booster shot,” said FDA vaccine chief Peter Marks. He added that for the tens of millions of Americans who have not yet been vaccinated against the coronavirus, “It’s not too late to start to get vaccinated.”
A third dose was also approved by the FDA for kids ages 5-11 who are certain levels of immunocompromised. The shot should be given at least 28 days following the second.
The FDA also reduced the required waiting period for a booster from six to five months after the second dose for anyone 12-years-old and older. Still, the Centers for Disease Control and Prevention needs to sign off on the authorization.
Health care systems are currently reporting record hospitalizations among children during a coronavirus surge, but as chief White House medical adviser Dr. Anthony Fauci noted, those numbers reflect children hospitalized with the coronavirus, not because of it.
Dr. Brian Monahan, Congress’ top doctor, urged lawmakers on Monday to shift to a “maximal telework posture,” due to a surge in COVID-19 cases at the Capitol, which he says are mostly breakthrough infections.
Monahan wrote in a letter to congressional leaders that the seven-day average rate of infection at the Capitol’s testing site has jumped from less than 1 percent to over 13 percent.
-

 Accountability3 days ago
Accountability3 days agoWaste of the Day: Principal Bought Lobster with School Funds
-

 Constitution2 days ago
Constitution2 days agoTrump, Canada, and the Constitutional Problem Beneath the Bridge
-
 Executive1 day ago
Executive1 day agoHow Relaxed COVID-Era Rules Fueled Minnesota’s Biggest Scam
-

 Civilization4 hours ago
Civilization4 hours agoWhy Europe Shouldn’t Be Upset at Trump’s Venezuelan Actions
-

 Civilization1 day ago
Civilization1 day agoThe End of Purple States and Competitive Districts
-

 Christianity Today3 hours ago
Christianity Today3 hours agoSurprising Revival: Gen Z Men & Highly Educated Lead Return to Religion
-

 Civilization5 days ago
Civilization5 days agoThe devil is in the details
-

 Executive4 days ago
Executive4 days agoTwo New Books Bash Covid Failures

