Accountability
FDA authorizes Pfizer booster shots for 12- to 15-year-olds
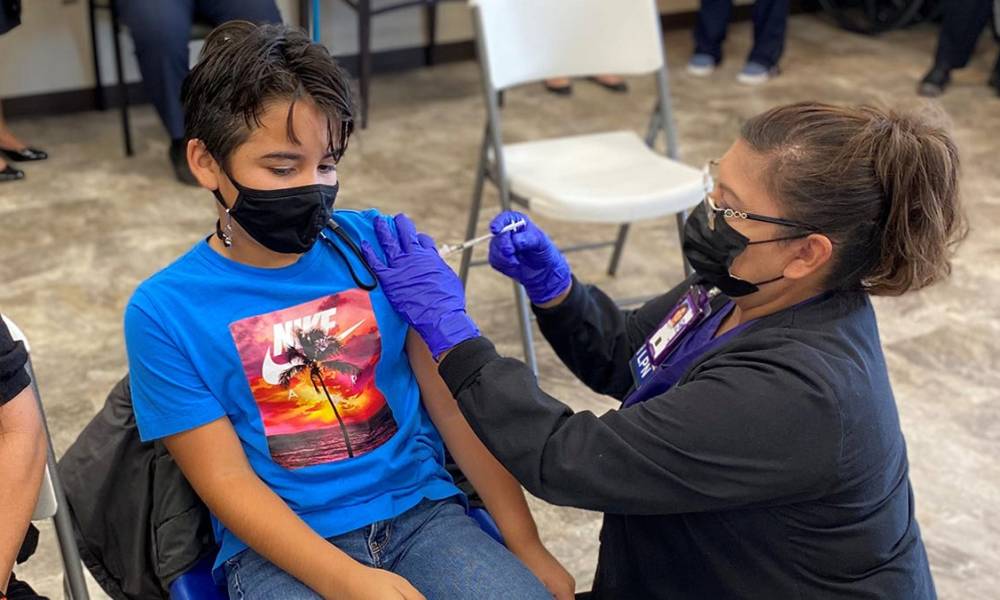
On Monday, the Food and Drug Administration expanded authorization for emergency use of a booster dose of the Pfizer-BioNTech coronavirus vaccine so that it now includes 12- to 15-year-olds.
“Hopefully this will be not just a call for people to get their booster shot,” said FDA vaccine chief Peter Marks. He added that for the tens of millions of Americans who have not yet been vaccinated against the coronavirus, “It’s not too late to start to get vaccinated.”
A third dose was also approved by the FDA for kids ages 5-11 who are certain levels of immunocompromised. The shot should be given at least 28 days following the second.
The FDA also reduced the required waiting period for a booster from six to five months after the second dose for anyone 12-years-old and older. Still, the Centers for Disease Control and Prevention needs to sign off on the authorization.
Health care systems are currently reporting record hospitalizations among children during a coronavirus surge, but as chief White House medical adviser Dr. Anthony Fauci noted, those numbers reflect children hospitalized with the coronavirus, not because of it.
Dr. Brian Monahan, Congress’ top doctor, urged lawmakers on Monday to shift to a “maximal telework posture,” due to a surge in COVID-19 cases at the Capitol, which he says are mostly breakthrough infections.
Monahan wrote in a letter to congressional leaders that the seven-day average rate of infection at the Capitol’s testing site has jumped from less than 1 percent to over 13 percent.
-

 Executive2 days ago
Executive2 days agoJanuary 6 case comes down to selective prosecution
-

 Executive1 day ago
Executive1 day agoBiden ballot woes continue
-

 News3 days ago
News3 days agoRolling the Dice on Republicans: Has the Right Become Delusional?
-

 Executive2 days ago
Executive2 days agoWhy Fatal Police Shootings Aren’t Declining: Some Uncomfortable Facts
-
 Civilization2 days ago
Civilization2 days agoPresident Biden Must Not Encourage Illegal Mass Migration From Haiti
-

 Constitution2 days ago
Constitution2 days agoEquality Under the Law and Conflicts of Interest in New York
-

 Civilization3 days ago
Civilization3 days agoBiology, the Supreme Court, and truth
-

 Guest Columns2 days ago
Guest Columns2 days agoWhat Was Won in No Labels’ Crusade

