Accountability
Pfizer-BioNTech asks FDA to authorize COVID-19 vaccine for children under 5
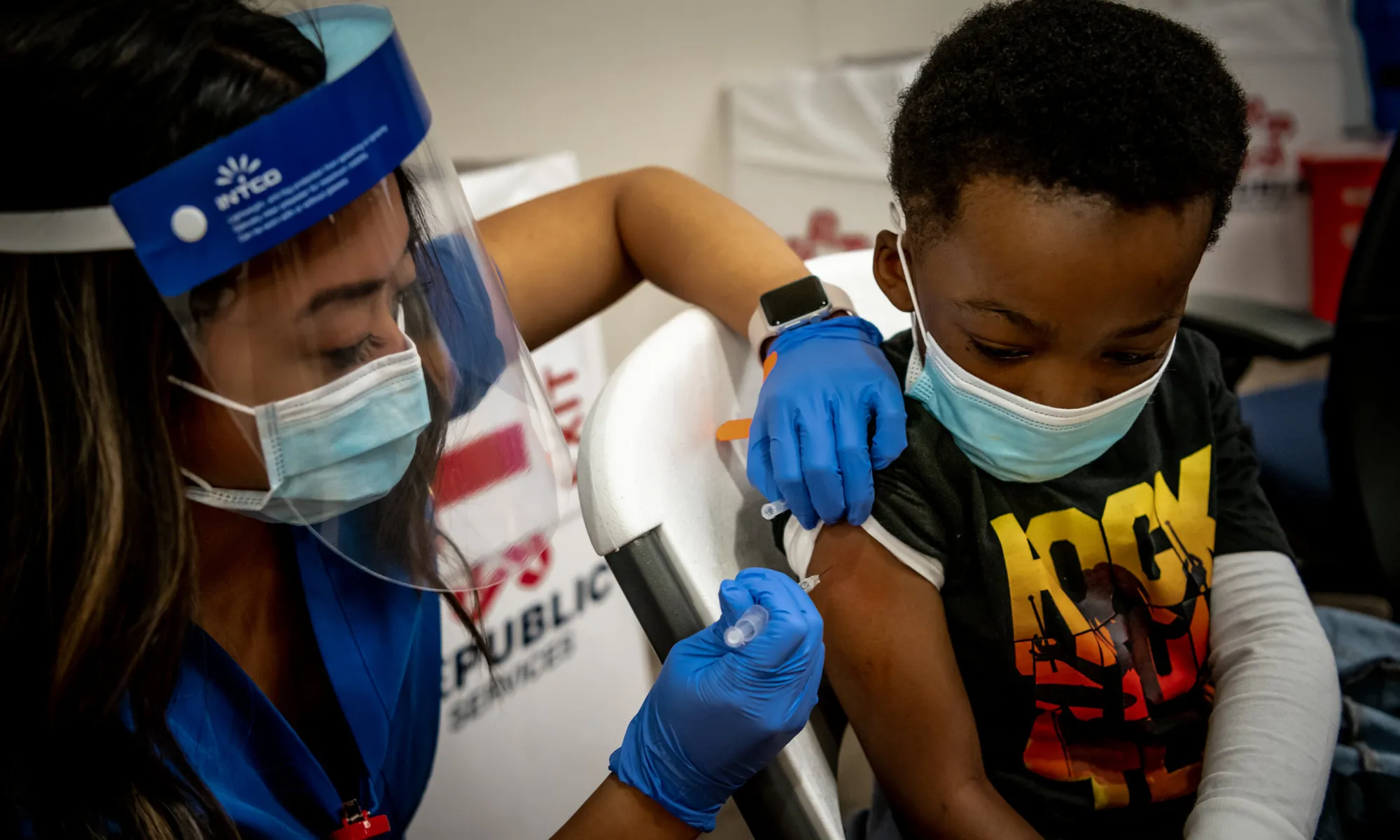
Pfizer-BioNTech is seeking to expand the availability of its COVID-19 vaccine by requesting authorization from the Food and Drug Administration for children ages five and under.
Data from a phase 2/3 trial of the vaccine included 1,678 children who had received a third dose during the period when the Omicron coronavirus variant dominated. Results of the trial were released May 23 and showed that the vaccine appeared to be safe and had a strong immune response. The data has not been peer-reviewed or published in a medical journal.
Antibody levels tested one month after the third dose showed that the vaccine produced a similar immune response as two doses in 16- to 25-year-olds, the companies said. Midtrial results found vaccine efficacy of 80.3% against symptomatic Covid-19 in this youngest age group.
The companies identified 10 symptomatic cases at least seven days after the third dose. However, the efficacy rate won’t be finalized until at least 21 symptomatic cases are found in the vaccine group and then compared with the number of symptomatic cases in the placebo group.
The vaccines for this youngest age group are smaller than those used in older groups. People 12 and older receive two doses of a 30-microgram vaccine, and children 5 to 12 receive two doses of a 10-microgram vaccine. Both of those groups are eligible for booster doses.
For children 6 months to 5 years, the Pfizer/BioNTech vaccine is three 3-microgram doses. The initial two doses were given three weeks apart, and the third dose was given at least two months after the second dose.
“These topline safety, immunogenicity and efficacy data are encouraging, and we look forward to soon completing our submissions to regulators globally with the hope of making this vaccine available to younger children as quickly as possible, subject to regulatory authorization,” Pfizer Chairman and CEO Albert Bourla said in a statement last month.
A vaccine for children between the ages of 6 months and 4 years, the only group still ineligible to get vaccinated against COVID-19, could be granted emergency use authorization by the FDA for the age group later this month.
The FDA’s advisory group, called the Vaccines and Related Biological Products Advisory Committee, is scheduled to meet June 15 to offer guidance on the pediatric doses.
Terry A. Hurlbut has been a student of politics, philosophy, and science for more than 35 years. He is a graduate of Yale College and has served as a physician-level laboratory administrator in a 250-bed community hospital. He also is a serious student of the Bible, is conversant in its two primary original languages, and has followed the creation-science movement closely since 1993.
-

 Accountability3 days ago
Accountability3 days agoWaste of the Day: Principal Bought Lobster with School Funds
-
 Executive1 day ago
Executive1 day agoHow Relaxed COVID-Era Rules Fueled Minnesota’s Biggest Scam
-

 Civilization12 hours ago
Civilization12 hours agoWhy Europe Shouldn’t Be Upset at Trump’s Venezuelan Actions
-

 Constitution2 days ago
Constitution2 days agoTrump, Canada, and the Constitutional Problem Beneath the Bridge
-

 Civilization1 day ago
Civilization1 day agoThe End of Purple States and Competitive Districts
-

 Christianity Today11 hours ago
Christianity Today11 hours agoSurprising Revival: Gen Z Men & Highly Educated Lead Return to Religion
-

 Executive24 hours ago
Executive24 hours agoWaste of the Day: Can You Hear Me Now?
-

 Executive4 days ago
Executive4 days agoTwo New Books Bash Covid Failures


