Accountability
FDA authorizes COVID vaccinations for ’emergency use’ in children under 5
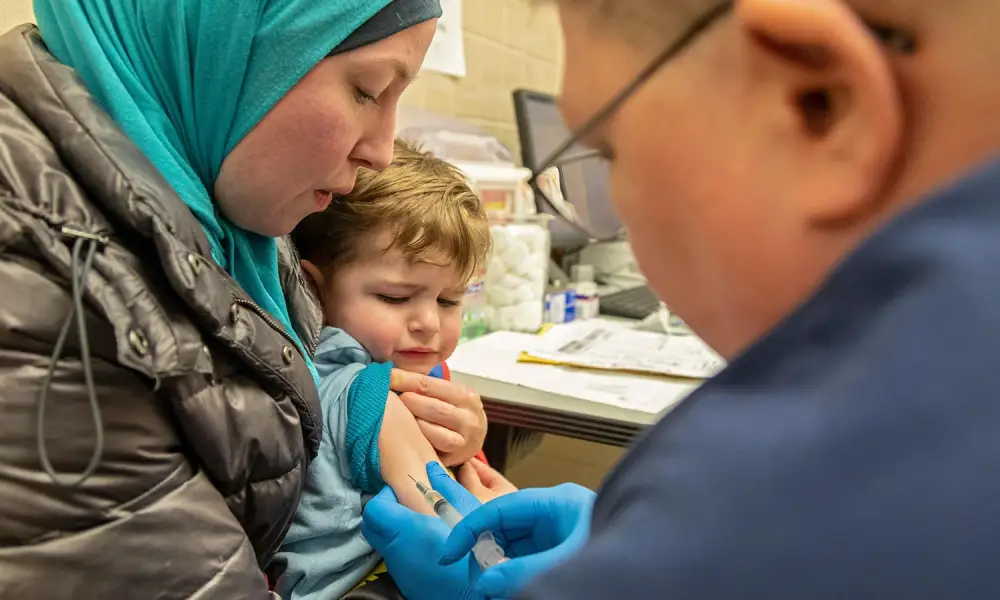
The US Food and Drug Administration (FDA) has authorized the first Covid-19 vaccines for children under age 5.
On Friday morning, the FDA granted emergency use for the Moderna and Pfizer jabs for children as young as 6 months.
“Many parents, caregivers and clinicians have been waiting for a vaccine for younger children and this action will help protect those down to 6 months of age,” stated FDA Commissioner Dr. Robert Califf. “As we have seen with older age groups, we expect that the vaccines for younger children will provide protection from the most severe outcomes of Covid-19, such as hospitalization and death.”
According to the FDA, immune responses of children 5 through 11 years of age were comparable to those of individuals 16 through 25 years of age. In addition, the vaccine was found to be 90.7% effective in preventing COVID-19 in children 5 through 11.
The vaccine’s safety was studied in approximately 3,100 children age 5 through 11 who received the vaccine and no serious side effects have been detected in the ongoing study.
The Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices will meet next week to discuss further clinical recommendations.
In the U.S., COVID-19 cases in children 5 through 11 years of age make up 39% of cases in individuals younger than 18 years of age. According to the CDC, approximately 8,300 COVID-19 cases in children 5 through 11 years of age resulted in hospitalization. As of Oct. 17,691 deaths from COVID-19 have been reported in the U.S. in individuals less than 18 years of age, with 146 deaths in the 5 through 11 years age group, per the FDA.
“The FDA is committed to making decisions that are guided by science that the public and healthcare community can trust. We are confident in the safety, effectiveness and manufacturing data behind this authorization. As part of our commitment to transparency around our decision-making, which included our public advisory committee meeting earlier this week, we have posted documents today supporting our decision and additional information detailing our evaluation of the data will be posted soon. We hope this information helps build confidence of parents who are deciding whether to have their children vaccinated,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research.
According to a Kaiser Family Foundation’s Vaccine Monitor survey, published in May, only 18% of parents of children under 5 said they would vaccinate their child against COVID-19 as soon as a vaccine was available.
“I think the more the pandemic is in the rearview mirror for some people, or they believe it is, then the less compelled they will be to do this, and so we have a big public health education campaign ahead of us,” Freeman said. “Also, health departments at the local level will be looking to understand the landscape of their community in terms of how many providers, pediatricians and pharmacies have actually signed up to give out the vaccine.”
-

 Executive3 days ago
Executive3 days agoThe Hunters Have Now Become The Hunted: Their Cruelties Are Swelling The Ranks Of The People Worldwide!
-

 Constitution3 days ago
Constitution3 days agoCHAPTER 9: Norman Dodd Interview Space Is No Longer the Final Frontier––Reality Is [upcoming release April 2024]
-

 Clergy2 days ago
Clergy2 days agoWhy Do The American People Let The Corrupt Media & Politicians Set The Propaganda Narrative – Speak On Their Behalf
-

 Executive4 days ago
Executive4 days agoBiden ballot woes continue
-
![CHAPTER 10: Objective Reality Is Required for a Free Society Space Is No Longer the Final Frontier—Reality Is [upcoming release May 2024]](https://cnav.news/wp-content/uploads/2024/04/Objective-reality-v-acceptance-400x240.png)
![CHAPTER 10: Objective Reality Is Required for a Free Society Space Is No Longer the Final Frontier—Reality Is [upcoming release May 2024]](https://cnav.news/wp-content/uploads/2024/04/Objective-reality-v-acceptance-80x80.png) Education2 days ago
Education2 days agoCHAPTER 10: Objective Reality Is Required for a Free Society Space Is No Longer the Final Frontier—Reality Is [upcoming release May 2024]
-

 Entertainment Today2 days ago
Entertainment Today2 days agoCivil War (2024) – an incomplete prediction
-

 Executive5 days ago
Executive5 days agoWhy Fatal Police Shootings Aren’t Declining: Some Uncomfortable Facts
-
 Civilization5 days ago
Civilization5 days agoPresident Biden Must Not Encourage Illegal Mass Migration From Haiti

