Accountability
FDA authorizes COVID vaccinations for ’emergency use’ in children under 5
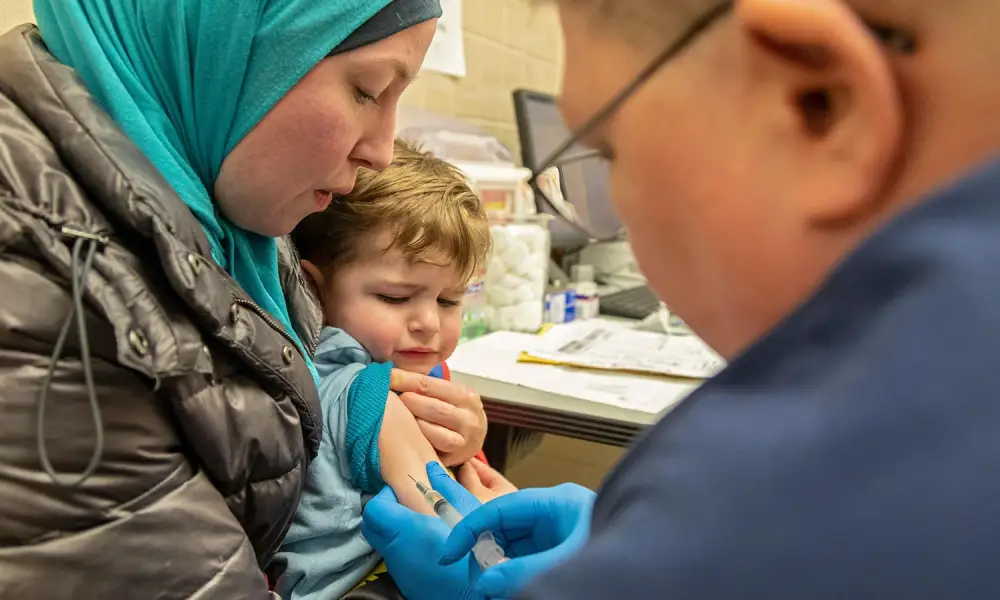
The US Food and Drug Administration (FDA) has authorized the first Covid-19 vaccines for children under age 5.
On Friday morning, the FDA granted emergency use for the Moderna and Pfizer jabs for children as young as 6 months.
“Many parents, caregivers and clinicians have been waiting for a vaccine for younger children and this action will help protect those down to 6 months of age,” stated FDA Commissioner Dr. Robert Califf. “As we have seen with older age groups, we expect that the vaccines for younger children will provide protection from the most severe outcomes of Covid-19, such as hospitalization and death.”
According to the FDA, immune responses of children 5 through 11 years of age were comparable to those of individuals 16 through 25 years of age. In addition, the vaccine was found to be 90.7% effective in preventing COVID-19 in children 5 through 11.
The vaccine’s safety was studied in approximately 3,100 children age 5 through 11 who received the vaccine and no serious side effects have been detected in the ongoing study.
The Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices will meet next week to discuss further clinical recommendations.
In the U.S., COVID-19 cases in children 5 through 11 years of age make up 39% of cases in individuals younger than 18 years of age. According to the CDC, approximately 8,300 COVID-19 cases in children 5 through 11 years of age resulted in hospitalization. As of Oct. 17,691 deaths from COVID-19 have been reported in the U.S. in individuals less than 18 years of age, with 146 deaths in the 5 through 11 years age group, per the FDA.
“The FDA is committed to making decisions that are guided by science that the public and healthcare community can trust. We are confident in the safety, effectiveness and manufacturing data behind this authorization. As part of our commitment to transparency around our decision-making, which included our public advisory committee meeting earlier this week, we have posted documents today supporting our decision and additional information detailing our evaluation of the data will be posted soon. We hope this information helps build confidence of parents who are deciding whether to have their children vaccinated,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research.
According to a Kaiser Family Foundation’s Vaccine Monitor survey, published in May, only 18% of parents of children under 5 said they would vaccinate their child against COVID-19 as soon as a vaccine was available.
“I think the more the pandemic is in the rearview mirror for some people, or they believe it is, then the less compelled they will be to do this, and so we have a big public health education campaign ahead of us,” Freeman said. “Also, health departments at the local level will be looking to understand the landscape of their community in terms of how many providers, pediatricians and pharmacies have actually signed up to give out the vaccine.”
-

 Accountability2 days ago
Accountability2 days agoWaste of the Day: Principal Bought Lobster with School Funds
-

 Constitution2 days ago
Constitution2 days agoTrump, Canada, and the Constitutional Problem Beneath the Bridge
-
 Executive23 hours ago
Executive23 hours agoHow Relaxed COVID-Era Rules Fueled Minnesota’s Biggest Scam
-

 Civilization22 hours ago
Civilization22 hours agoThe End of Purple States and Competitive Districts
-

 Civilization4 days ago
Civilization4 days agoThe devil is in the details
-

 Executive4 days ago
Executive4 days agoTwo New Books Bash Covid Failures
-

 Civilization4 days ago
Civilization4 days agoThe Conundrum of President Donald J. Trump
-

 Executive4 days ago
Executive4 days agoThe Israeli Lesson Democrats Ignore at Their Peril

