News
Infant RSV vaccine could be FDA approved by end of summer
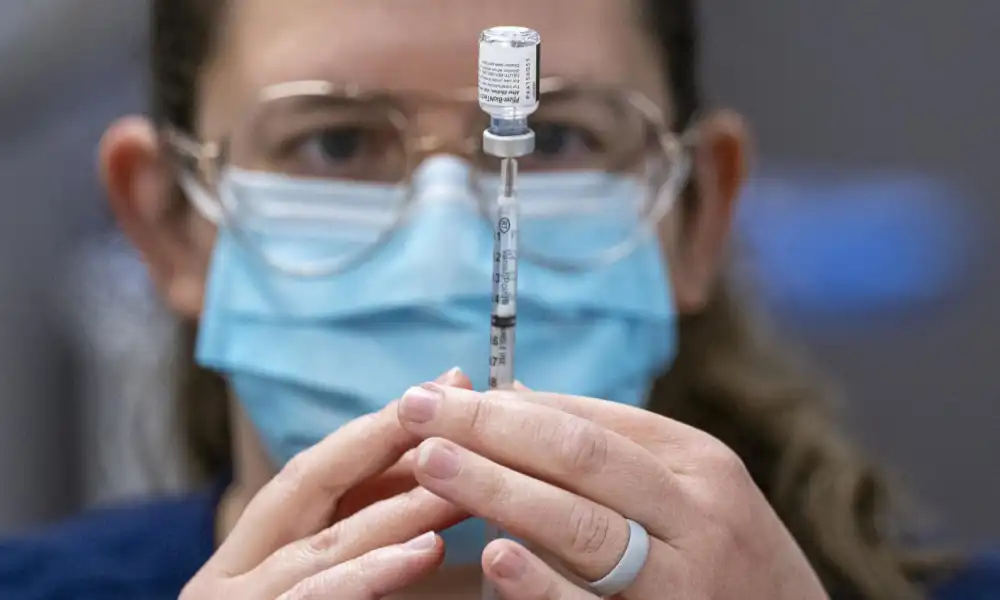
A new Pfizer vaccine to prevent respiratory syncytial virus (RSV) in infants could be approved by the Food and Drug Administration by the end of the summer, according to officials.
The vaccine, once approved and available to the public, would be administered to the pregnant mother while the unborn baby was in utero. The vaccine would then pass through the placenta and protect the baby from RSV once born. The United States has seen a major surge in positive cases of RSV since the lifting of COVID-19 restrictions that prevented the transfer of RSV for some time.
Pfizer confirmed this week that the Food and Drug Administration is currently conducting an expedited review of the RSV vaccine that could wrap up in time to approve the vaccine by the end of August, ahead of the annual RSV surge typically seen in the fall months in the US. “U.S. FDA has set an action date for August 2023,” wrote the pharmaceutical company. “If approved, RSVpreFwould be the first vaccine for administration to pregnant individuals to help protect against the complications of RSV disease in infants from birth through six months.”
In clinical trials, Pfizer reported an 82 percent protection rate for infants against RSV when issuing the vaccine to pregnant mothers.
Terry A. Hurlbut has been a student of politics, philosophy, and science for more than 35 years. He is a graduate of Yale College and has served as a physician-level laboratory administrator in a 250-bed community hospital. He also is a serious student of the Bible, is conversant in its two primary original languages, and has followed the creation-science movement closely since 1993.
-

 Executive1 day ago
Executive1 day agoJanuary 6 case comes down to selective prosecution
-

 News2 days ago
News2 days agoRolling the Dice on Republicans: Has the Right Become Delusional?
-

 Civilization2 days ago
Civilization2 days agoBiology, the Supreme Court, and truth
-
 Civilization15 hours ago
Civilization15 hours agoPresident Biden Must Not Encourage Illegal Mass Migration From Haiti
-

 Executive17 hours ago
Executive17 hours agoWhy Fatal Police Shootings Aren’t Declining: Some Uncomfortable Facts
-

 Guest Columns16 hours ago
Guest Columns16 hours agoWhat Was Won in No Labels’ Crusade
-

 Entertainment Today18 hours ago
Entertainment Today18 hours agoWaste of the Day: Throwback Thursday: Millions Went To Video Game ‘Research’
-

 Constitution16 hours ago
Constitution16 hours agoEquality Under the Law and Conflicts of Interest in New York


