Accountability
FDA restricts Johnson & Johnson COVID vaccine for some due to blood clot risk
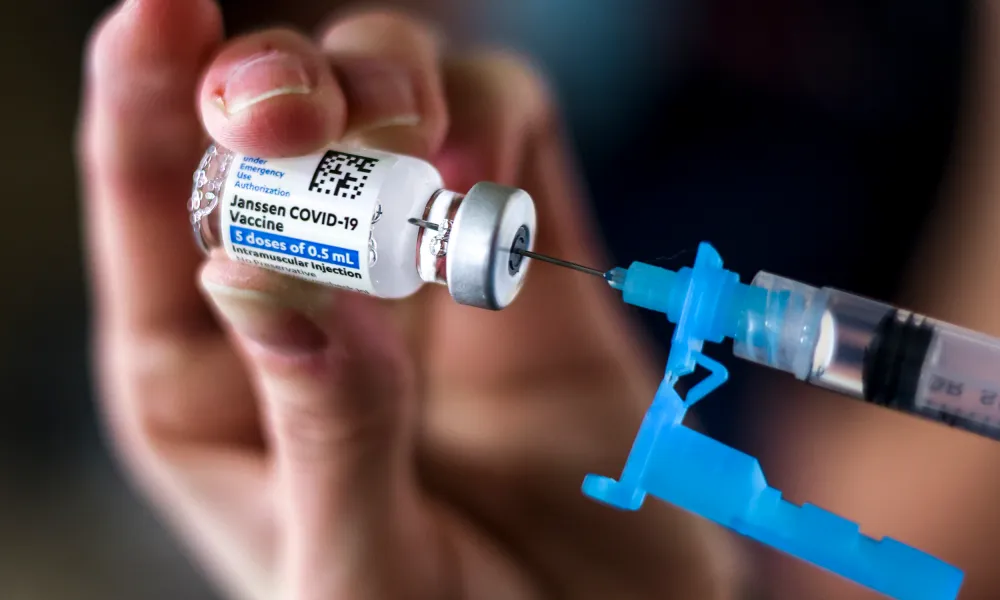
On Thursday the U.S. Food and Drug Administration has limited the authorized use of the Janssen COVID-19 vaccine to individuals 18 years of age and older for whom other authorized or approved COVID-19 vaccines are not accessible or clinically appropriate, and to individuals 18 years of age and older who elect to receive the Janssen COVID-19 Vaccine because they would otherwise not receive a COVID-19 Vaccine.
After conducting an updated analysis, evaluation and investigation of reported cases, the FDA has determined that the risk of thrombosis with thrombocytopenia syndrome (TTS), a syndrome of rare and potentially life-threatening blood clots in combination with low levels of blood platelets with onset of symptoms approximately one to two weeks following administration of the Janssen COVID-19 Vaccine, warrants limiting the authorized use of the vaccine.
The FDA has determined that the known and potential benefits of the vaccine for the prevention of COVID-19 outweigh the known and potential risks for individuals 18 years of age and older for whom other authorized or approved COVID-19 vaccines are not accessible or clinically appropriate, and for individuals 18 years of age and older who elect to receive the Janssen COVID-19 Vaccine because they would otherwise not receive a COVID-19 vaccine. (FDA website)
“We recognize that the Janssen COVID-19 Vaccine still has a role in the current pandemic response in the United States and across the global community. Our action reflects our updated analysis of the risk of TTS following administration of this vaccine and limits the use of the vaccine to certain individuals,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research.
“Today’s action demonstrates the robustness of our safety surveillance systems and our commitment to ensuring that science and data guide our decisions. We’ve been closely monitoring the Janssen COVID-19 Vaccine and occurrence of TTS following its administration and have used updated information from our safety surveillance systems to revise the EUA. The agency will continue to monitor the safety of the Janssen COVID-19 Vaccine and all other vaccines, and as has been the case throughout the pandemic, will thoroughly evaluate new safety information.”
A Johnson & Johnson spokesman said in an emailed statement: “Data continue to support a favorable benefit-risk profile for the Johnson & Johnson COVID-19 vaccine in adults, when compared with no vaccine.”
Terry A. Hurlbut has been a student of politics, philosophy, and science for more than 35 years. He is a graduate of Yale College and has served as a physician-level laboratory administrator in a 250-bed community hospital. He also is a serious student of the Bible, is conversant in its two primary original languages, and has followed the creation-science movement closely since 1993.
-

 Accountability3 days ago
Accountability3 days agoWaste of the Day: Principal Bought Lobster with School Funds
-

 Civilization17 hours ago
Civilization17 hours agoWhy Europe Shouldn’t Be Upset at Trump’s Venezuelan Actions
-
 Executive2 days ago
Executive2 days agoHow Relaxed COVID-Era Rules Fueled Minnesota’s Biggest Scam
-

 Constitution3 days ago
Constitution3 days agoTrump, Canada, and the Constitutional Problem Beneath the Bridge
-

 Christianity Today17 hours ago
Christianity Today17 hours agoSurprising Revival: Gen Z Men & Highly Educated Lead Return to Religion
-

 Civilization2 days ago
Civilization2 days agoThe End of Purple States and Competitive Districts
-

 Executive1 day ago
Executive1 day agoWaste of the Day: Can You Hear Me Now?
-

 Civilization4 days ago
Civilization4 days agoThe Conundrum of President Donald J. Trump


