News
FDA authorizes updated COVID-19 booster shots
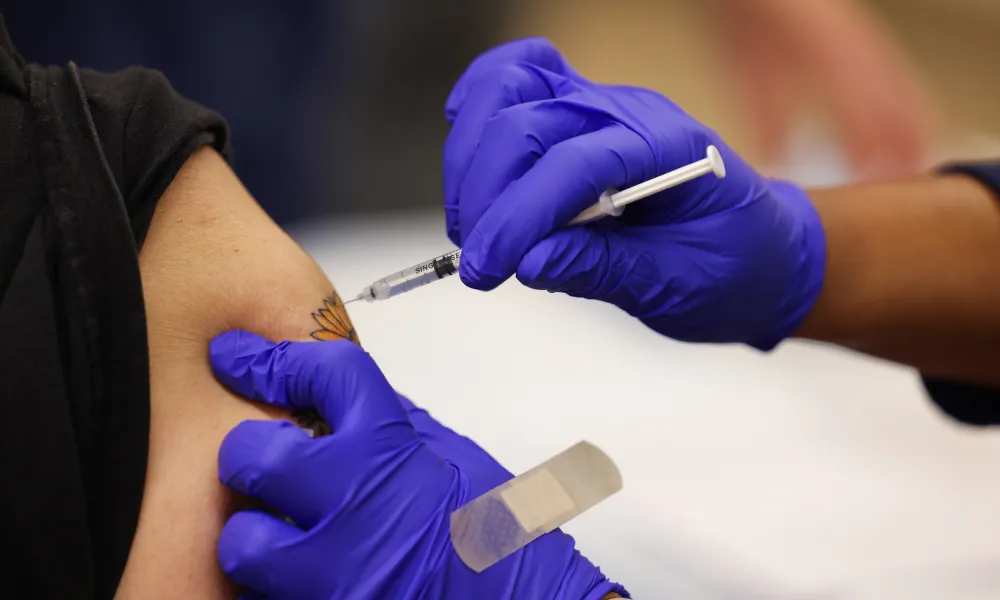
The U.S. Food and Drug Administration (FDA) announced on Wednesday that it has authorized updated coronavirus booster shots which are specifically designed for the new omicron variant, which is said to be more contagious.
“The BA.4 and BA.5 lineages of the omicron variant are currently causing most cases of COVID-19 in the U.S. and are predicted to circulate this fall and winter,” the FDA said. “Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUAs) of the Moderna COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine to authorize bivalent formulations of the vaccines for use as a single booster dose at least two months following primary or booster vaccination.”
The new shots, which the FDA is referring to as “updated boosters,” contain “two messenger RNA (mRNA) components of SARS-CoV-2 virus, one of the original strain of SARS-CoV-2 and the other one in common between the BA.4 and BA.5 lineages of the omicron variant of SARS-CoV-2,” the statement went on to say.
FDA commissioner Robert Califf provided more details in a separate statement: “The COVID-19 vaccines, including boosters, continue to save countless lives and prevent the most serious outcomes (hospitalization and death) of COVID-19. As we head into fall and begin to spend more time indoors, we strongly encourage anyone who is eligible to consider receiving a booster dose with a bivalent COVID-19 vaccine to provide better protection against currently circulating variants.”
The FDA added that Moderna boosters are authorized for use with adults over the age of 18 whereas the Pfizer-BioNTech booster can be used on anyone over the age of 12.
The FDA has promised it will “will work quickly to evaluate future data and submissions to support authorization of bivalent COVID-19 boosters for additional age groups as we receive them.”
Peter Marks, who is the director of the FDA’s Center for Biologics Evaluation and Research, confirmed that the FDA has anticipated for some time that further variants would have made its way into circulation.
“The FDA has been planning for the possibility that the composition of the COVID-19 vaccines would need to be modified to address circulating variants. We sought input from our outside experts on the inclusion of an omicron component in COVID-19 boosters to provide better protection against COVID-19. We have worked closely with the vaccine manufacturers to ensure the development of these updated boosters was done safely and efficiently,” Marks said.
Terry A. Hurlbut has been a student of politics, philosophy, and science for more than 35 years. He is a graduate of Yale College and has served as a physician-level laboratory administrator in a 250-bed community hospital. He also is a serious student of the Bible, is conversant in its two primary original languages, and has followed the creation-science movement closely since 1993.
-

 Civilization2 days ago
Civilization2 days agoWhy Europe Shouldn’t Be Upset at Trump’s Venezuelan Actions
-

 Accountability4 days ago
Accountability4 days agoWaste of the Day: Principal Bought Lobster with School Funds
-
 Executive3 days ago
Executive3 days agoHow Relaxed COVID-Era Rules Fueled Minnesota’s Biggest Scam
-

 Constitution4 days ago
Constitution4 days agoTrump, Canada, and the Constitutional Problem Beneath the Bridge
-

 Christianity Today2 days ago
Christianity Today2 days agoSurprising Revival: Gen Z Men & Highly Educated Lead Return to Religion
-

 Civilization3 days ago
Civilization3 days agoThe End of Purple States and Competitive Districts
-

 Executive3 days ago
Executive3 days agoWaste of the Day: Can You Hear Me Now?
-

 Executive3 days ago
Executive3 days agoWaste of the Day: States Spent Welfare in “Crazy Ways”


