Judicial
Mifepristone before 5th Circuit
The Texas Mifepristone Case came to oral argument before the Fifth Circuit Court of Appeals, with many expecting a ruling against the drug.
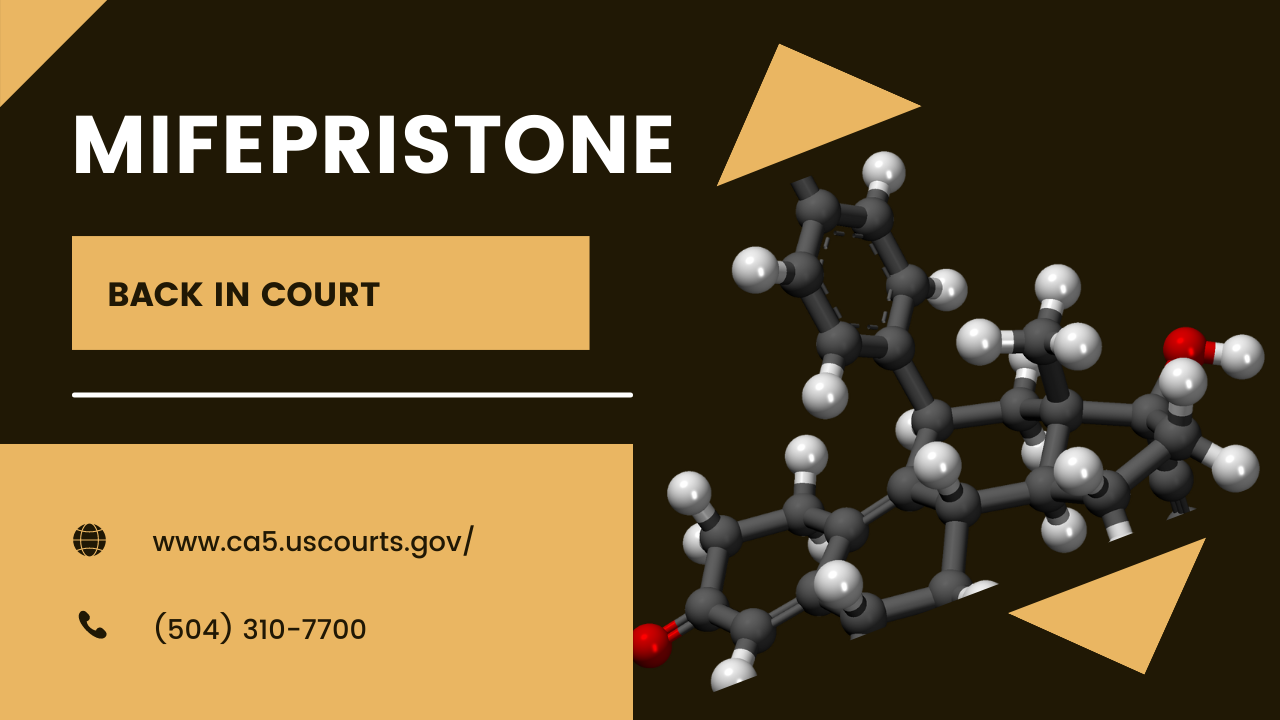
The case involving the availability (and regulation) of mifepristone, a chemical abortifacient, came to oral argument before an appeals court.
Mifepristone comes to argument
A three-judge panel of the Court of Appeals for the Fifth Judicial Circuit heard argument on the “Mifepristone Case.” (Alliance for Hippocratic Medicine et al. v. U.S. Food and Drug Administration et al.) The Texas Tribune and Insider Paper carried the story. The Fifth Circuit Court maintains a YouTube channel and made the two-hour oral argument session available here:
Judges Jennifer Walker Elrod, James C. Ho, and Cory T. Wilson formed the panel. Judge Elrod has served the longest; George W. Bush nominated her to the Court. President Donald Trump nominated the other two.
The government appeared to argue that the FDA were experts, and courts should defer to them because they were experts. Judge Ho rejected that argument out-of-hand, saying, “I don’t understand this idea that FDA can do no wrong.” He went on, “We are allowed to look at FDA just like any other agency. That’s the role of the courts.”
History of the case
The case is in the Fifth Circuit after Judge Matthew Kacsmaryk suspended the FDA’s original approval of mifepristone, as well as the FDA’s subsequent loosening of the regulations governing its use. The Fifth Circuit held that any challenge to the 2000 approval of the drug is “time barred.” Which means the statute of limitations for such a challenge has run out. But the Fifth Circuit held that the FDA’s actions from 2016 onwards are not subject to the same time bar. They also said then that the plaintiffs are likely to prevail on the merits. However the Supreme Court stayed all restrictions on mifepristone pending the disposition of the case by the Fifth Circuit.
The plaintiffs come to the Fifth Circuit trying to reverse the “time bar” determination on the 2000 approval. Judge Kacsmaryk found that the plaintiffs have been challenging the 2000 approval since 2002, and that:
Before Plaintiffs filed this case, FDA ignored their petitions for over sixteen years, even though the law requires an agency response within “180 days of receipt of the petition.” 21 C.F.R. § 10.30(e)(2)). But FDA waited 4,971 days to adjudicate Plaintiffs’ first petition and 994 days to adjudicate the second. See ECF Nos. 1-14, 1-28, 1-36, 1-44 (“2002 Petition,” “2019 Petition,” respectively). Had FDA responded to Plaintiffs’ petitions within the 360 total days allotted, this case would have been in federal court decades earlier. Instead, FDA postponed and procrastinated for nearly 6,000 days.
The government opened by saying what Judge Kacsmaryk did was “unprecedented.” But the panel disputed that characterization at once, citing an FDA challenge the day before.
Jennifer Dalven, director of the Reproductive Freedom Project of the American Civil Liberties Union, expressed pessimism. While continuing to assert that the plaintiffs’ case has no merit, she lamented the composition of the panel.
The respondents have two options after the Fifth Circuit rules. They can either petition the Fifth Circuit to hear the case en banc or petition the Supreme Court directly.
Suppose SCOTUS rules against them?
Obviously looking ahead to a Supreme Court decision, Gov. Phil Murphy (D-N.J.) threatened open defiance of any decision against mifepristone. Breitbart described Murphy’s attitude – including his invocation of the Name of God in an interview. He spoke of bulk acquisition of mifepristone, and actually said a decision to ban it would “cost lives.” But he did not say how failure to prescribe mifepristone, or perform an abortion by any other means, would cost a woman’s life.
The Texas Tribune describes friend-of-the-court briefings suggesting that if the law (specifically the Comstock Act) forbids sending mifepristone through the mail, then it would also forbid the shipment of other devices, surgical instruments, and similar medical equipment. How that would result is not clear, since mifepristone has no use other than abortion, that other drugs or procedures cannot meet. That doesn’t apply to the other equipment that concerns those “friends of the court.”
Terry A. Hurlbut has been a student of politics, philosophy, and science for more than 35 years. He is a graduate of Yale College and has served as a physician-level laboratory administrator in a 250-bed community hospital. He also is a serious student of the Bible, is conversant in its two primary original languages, and has followed the creation-science movement closely since 1993.
-

 Accountability2 days ago
Accountability2 days agoWaste of the Day: Principal Bought Lobster with School Funds
-

 Constitution2 days ago
Constitution2 days agoTrump, Canada, and the Constitutional Problem Beneath the Bridge
-
 Executive20 hours ago
Executive20 hours agoHow Relaxed COVID-Era Rules Fueled Minnesota’s Biggest Scam
-

 Civilization20 hours ago
Civilization20 hours agoThe End of Purple States and Competitive Districts
-

 Civilization4 days ago
Civilization4 days agoThe devil is in the details
-

 Executive4 days ago
Executive4 days agoTwo New Books Bash Covid Failures
-

 Civilization3 days ago
Civilization3 days agoThe Conundrum of President Donald J. Trump
-

 Executive4 days ago
Executive4 days agoThe Israeli Lesson Democrats Ignore at Their Peril















The fact that the FDA was challenged in the 2002 time frame and ignored the challenge invalidates any of the claims that the challenges are too late. And those who work to kill children, before and after birth, will lie to advance their agenda.